- Animal health
- Animal Nutrition
- Genetic and Biodiversity
- Environment and natural resources protection
- Socio-economy in pig production sector
- Quality and food safety
- Animal husbandry and sustainable practices
- Rural Development
African Swine Fever: outbreak in Nigeria | Field and Experimental Investigations of an Outbreak of African Swine Fever
An outbreak of ASF, characterized by a mortality of 50 to 100% in various herds, was diagnosed among free-ranging domesticated pigs in Delta State, Nigeria, in August 1998. Here are detailed the results of the etiological confirmation and postmortem examination.
An outbreak of African Swine Fever (ASF), characterized by a mortality of 50 to 100% in various herds, was diagnosed among free-ranging domesticated pigs in Delta State, Nigeria, in August 1998. The etiological confirmation of ASF was made by virus isolation, PCR and sequencing of a 280 base pair fragment of the major capsid protein (VP72) gene. Experimental infection of pigs with infected blood resulted in pyrexia, which peaked two to four days postinfection, followed by death in five to six days postinfection. Postmortem examination revealed widespread hemorrhage, congestion and edema of tissues. The lymph nodes, spleen, liver and kidneys showed marked focal random necrosis and loss of lymphocytes from the splenic and lymphoid follicles. There was an acute orchitis with massive neutrophilic and macrophage infiltrates into the intertubular connective tissue. Meningitis and focal hemorrhages were observed in the brain and spinal cord. The outbreak was believed to be a continuation of an eastward spread of ASF from neighboring Benin, which began the previous year (1997).
Introduction
The African Swine Fever (ASF), caused by a deoxyribonucleic acid (DNA) virus belonging to the Asfarviridae family, is a highly contagious viral disease of domesticated pigs, characterized by widespread hemorrhages and very high mortality (11). Although the disease tends to run a subacute or chronic course in areas where it has become enzootic, mortality rates close to 100% have often been observed during epizootics in previously free areas (15). Following the first report of the disease in Kenya (9), it was subsequently reported in South Africa (4) and Angola (16). With the exception of Lesotho and Swaziland, ASF has been reported in all countries of Southern, Eastern and Central Africa (14, 17). In West Africa, ASF outbreaks have been recorded in Cameroon, Cape Verde islands, Guinea Bissau, Senegal and Cote d’Ivoire (14). Between 1997 and 1998, ASF outbreaks occurred in Togo, Benin and Nigeria (14).
The first outbreak in Nigeria, which occurred in September 1997, was associated with a spread of the epizootic from Benin. Thus, an unprecedented high rate of mortality among pigs was reported from four local government areas of Ogun State (Ipokia, Yewah- South, Yewa-North, and Imeko-Afon), bordering Benin. An ASF outbreak also occurred in the contiguous Lagos State from the end of 1997 to early 1998 (13). Then, a second wave of ASF outbreak started about in August 1998 and involved six states of the Nigerian federation, namely Kaduna and Benue States (in the north), and Enugu, Akwa Ibom, Rivers and Delta States (in the south). The present paper reports the investigation of the ASF outbreak in Delta State, Nigeria, in August 1998.
Materials and methods
Case report
Assistance was sought from the Veterinary Teaching Hospital, University of Ibadan, for the investigation of the cause of widespread mortality among free-roaming domesticated pigs in Warri North local government area of Delta State, Nigeria, in August 1998. Pig rearers reported a suspected case of poisoning as a result of environmental pollution following crude oil spillage from the facilities of an oil prospecting company operating in the area.
The outbreak area, visited between August 27 and 29, 1998, consisted of small settlements around the estuary of the Benin River, on the coastline of the Atlantic ocean and along canals dredged by the oil prospecting company (Figure 1). The area lies within longitudes 4°50’ E and 5°15’ E, and latitudes 5°45’ N and 6° N. All the affected communities were linked by branches of the Benin River and a network of canals. Most of the pigs were free-roaming and of indigenous variety. They were maintained on grass, supplemented with cassava chaff and swill from the kitchens of the facilities servicing the oil prospecting company.
Pigs’ deaths were said to have started in the area of the outbreak around the first week of August 1998. Affected pigs were noticed to be off-feed, weak and frequently lying down. Some pigs also manifested, either singly or combined, diarrhea, ocular discharges and a staggering gait. Death reportedly occurred following an illness of one to three days. At the time of the visit, the case fatality rate in most pig herds was 50 to 100%. At the Gbokoda community it involved three pig herds with populations of 15 to 20 pigs per herd, at Ilueri community one pig herd with a population of 25 pigs, and at Odonla community three pig herds with populations of 35 to 50 pigs per herd. It was observed that in some of the communities, dead pigs were thrown into waterways with the intention of preventing the spread of the disease to other animals in the area.
Sample collection
At Gbokoda, Ilueri and Odonla communities, blood samples were collected from sick pigs into vacutainer tubes containing EDTA. Samples were transported to the laboratory on wet ice and stored at 4°C until used. In the same communities, postmortem examination was conducted on six pigs. Samples of lymph nodes, spleen, liver, lungs, thyroid gland and kidneys were fixed in buffered formol saline for histopathology. A second set of tissue samples were collected into sterile glass containers and transported to the laboratory on wet ice.
Laboratory investigations
In the laboratory, histopathological, bacteriological, parasitological, and virological studies were carried out on the samples collected from the outbreak areas. Tissues for histopathology were processed routinely and stained with hematoxylin and eosin. Samples of the spleen, lymph nodes, lungs, kidney, liver and thyroid gland were inoculated in duplicate onto 5% sheep blood agar (Oxoid, Columbia, USA) and MacConkey agar (Oxoid). The cultures were incubated aerobically and anaerobically at 37°C and examined daily for bacterial growth. Bacterial colonies found on the cultures were examined by standard procedures (3, 6). Blood samples were screened for trypanosomosis by the hematocrit centrifuge technique (12), and smears made on microscopic glass slides were stained with Giemsa for the detection of other blood parasites.
Diagnostic polymerase chain reaction (PCR) using ASF virusspecific primer and virus isolation on a primary culture of swine peripheral blood mononuclear cells (SPBMC) were carried out as previously described (13). Briefly, 20 ml of blood were collected from the anterior vena cava of each of five intensively-reared Large White pigs, kept at the Teaching and Research Farm of the University of Ibadan, into sterile heparinized tubes. SPBMCs were isolated from the blood by Ficoll-Hypaque (Pharmacia, Piscataway, USA) isocentrifugation and washed three times with phosphate- buffered-saline (PBS). The cells were resuspended in Hank’s balanced salt solution and counted in a Neubauer’s chamber. SPBMCs were then resuspended in an RPMI-1640 culture medium containing 10% fetal bovine serum (FBS), penicillin–streptomycin, glutamine and amphotericin B (Fungizone®), to contain 105 cells per milliliter. Ten milliliters of cell suspension were seeded into a 50 cm3 of tissue culture flasks and incubated at 37°C in a humidified 5% CO2 incubator until the cells were confluent in about 72 h. Non-adherent cells were then decanted and adherent cells washed three times with warm sterile PBS and then kept in RPMI as a monolayer of the adherent SPBMCs used for virus isolation.
Pieces of the liver were homogenized with RPMI 1640 in a sterile mortar. The homogenized tissue was clarified by centrifugation at 5000 g and the supernatant filtered using a 0.45 ?m syringe filter (Nalgene, Rochester, USA). About 2.0 ml of the filtrate from the supernatant were then added to the adherent SPBMC culture and incubated at 37°C for 2 h for virus adsorption to occur. The unadsorbed inoculum was then rinsed three times with warm sterile PBS and 10 ml of RPMI-1640 maintenance medium added to the infected SPBMC. Thereafter, the culture was incubated at 37°C and observed daily for cytopathic changes. Hemadsorption assays to determine virus growth in the culture were performed on the infected cells and non-infected controls after five days essentially as described by Wilkinson et al. (18).
DNA was extracted from macerated liver and lung tissues and from cell culture supernatant by a standard phenol-chloroform extraction method followed by isopropanol precipitation. Using 5 ?l of the DNA sample, a diagnostic PCR was carried out in a 50 ?l reaction volume (1.5 mM MgCl2, 0.2 ?M) of each primer in 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 200 ?M of each deoxynucleotide triphosphate (dNTP), and 2.5 U of platinum Taq polymerase (Life Technologies, Grand Island, USA). Specific VP72 capsid primers were designed as follows: ASF 6155: 5’ CTT ACC GAT GAA AAT GAT AC 3’ (corresponding to position 772-791 in strain DR-1, Genbank accession number L27498), and antisense primer, ASF 6156: 5’ A (A/G)G GAT ACC GAG GGA ATA GC 3’ (corresponding to position 1047-1030 in strain DR-1). The 280 bp fragment from the diagnostic PCR was gel-purified using the concert rapid PCR product purification kit (GIBCO Life Technologies) and sequenced directly in both directions with ASF 6155 and ASF 6156 primers using the Applied Biosystems Dye Terminator Cycle Sequencing kit (Perkins- Elmer). The sequences were resolved on an ABI Prism 377 automatic sequencer (Applied Biosystems). A BLAST sequence similarity search (1) was carried out to compare the 98/ASF/NgDL 280bp sequence from VP72 gene with known sequences on the Genbank database. The virus was also compared with 98/ASF/Ng which was previously isolated from Lagos, Nigeria (13).

Figure 1: Area of the study involved in pigs’ deaths.
Experimental investigation
Six apparently healthy four-month-old Large White x Land Race pigs, obtained from a herd on the Teaching and Research Farm, University of Ibadan, were used for experimental investigation. The pigs were fed a commercial grower’s feed and given water ad libitum. They were divided into two groups housed separately: an experimental group consisting of four pigs, and a control group consisting of two pigs. Each of the four pigs in the experimental group was inoculated subcutaneously with 5 ml of a pool of refrigerated anticoagulated blood collected from sick pigs involved in the field outbreak, while the control pigs were inoculated with anticoagulated blood from non-infected pigs from the University Farm. Following inoculation, all the pigs were given intramuscular oxytetracycline injections (20 mg/kg) daily for three days postinoculation to forestall potential bacterial infection from the inoculum. The animals were monitored daily for temperature and other clinical signs until death. Four days postinfection, 5 ml of blood were collected from the anterior vena cava of each pig into vials with EDTA for virology. Virus isolation and identification were done as described above. Postmortem examination was conducted on all pigs within 12 h of death and tissues obtained for histopathology as described under the laboratory investigations above.
Results
Investigation of field cases
Postmortem examination revealed multifocal petechial skin hemorrhages occurring along the whole length of the ventral (abdominal) surface in one pig only. Grossly, the lymph nodes, liver, kidneys were congested and hemorrhagic. The lungs were also congested and edematous while the thyroid gland was gelatinous and glassy in appearance. Upon histology, the spleen, lymph nodes, kidneys, liver and thyroid gland showed marked hemorrhage in addition to necrosis. Bacterial colonies were observed in the spleen and lymph nodes of two pigs. The lung lesion was that of marked congestion and edema. No parasites were seen in the blood samples examined. While no growth was found on the bacterial cultures of liver, kidneys, and thyroid gland, Staphylococcus epidermidis was isolated from the spleen, lung and lymph nodes.
An agent, which adsorbed pig red blood cells, named 98/ASF/ NgDL, was isolated from the samples of blood, liver, spleen and lungs using SPBMC. PCR of DNA samples extracted from the liver, lungs, spleen and blood samples of infected pigs using primer pairs specific for the ASF virus (ASFV) resulted in the amplification of a 280 base pair genomic fragment of the virus. In addition, PCR was used to confirm the replication of ASFV in a culture of SPBMCs infected with homogenates of tissues of infected pigs. Sequencing of the PCR product led to the resolution of 200 bases from the 280 bp product without any ambiguity. While this fragment showed a 100% similarity to the isolate from Lagos, Nigeria, 98/ASF/Ng, it showed a similarity of 98% to the isolate from the Dominican Republic (DR-1) in the short region analyzed.
Experimental investigation
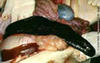
Experimentally-infected pigs showed an initial rise in body temperature by one day postinfection (p.i.), which peaked 2 to 4 days p.i. (Table I). By 3 to 4 days p.i., infected pigs tended to huddle together and refused to feed. Terminally infected pigs went into lateral recumbency with two of them showing labored respiration, jerky expiratory movements and discharge of clear fluid from the mouth and nostrils. Death of infected pigs occurred within 24 h of recumbency and at 5 to 6 days p.i. (Table I). The two uninfected pigs remained apparently healthy throughout the duration of the study. Postmortem examination of experimentally infected pigs showed that the snout, nasal turbinate, soft palate and thyroid gland were congested. There was excessive serosanguineous fluid with fibrin strands in the thoracic cavity of two pigs. The lungs were edematous, had a rubbery consistency, and showed a mosaic appearance with lobules being red or grey and depressed; the diaphragmatic lobes were most severely affected. Hydropericardium was observed in one pig while the pericardial sac of another contained about 10 ml of blood. The atria and ventricles of two pigs contained chicken fat clot, while the liver and spleen were grossly slightly congested with foci of necrosis. The kidneys and most lymph nodes were enlarged and edematous, especially the mesenteric lymph nodes. Both kidneys of one of the experimentally infected pigs had petechial hemorrhages on the cortical surface with congestion of the pelvis and medulla, while the kidney of another pig showed moderate subcapsular hemorrhage. The stomach of three of the experimentally infected pigs showed congestion with some areas of erosion and hemorrhage in the fundic and pyloric regions. Although the small intestine was also congested, the large intestine was more affected with areas of multifocal hemorrhage. Generally there was moderate congestion of the blood vessels of the brain. Details of the clinical findings and necropsy of experimentally infected pigs are shown in Tables II and III, respectively. At histology, the trachea, lungs, esophagus and intestines showed an acute inflammatory reaction characterized by vasculitis, congestion and edema with moderate neutrophilic and macrophage infiltrates. The lesion in the lungs was that of interstitial pneumonia with necrosis of bronchial associated lymphoid tissue. The liver, spleen (Figure 2), kidneys and lymph nodes showed marked focal random necrosis with hemorrhages and loss of lymphocytes from the splenic and lymphoid follicles in addition to edema and disseminated intravascular coagulation in the lymph nodes (Figure 3). The lesions in the testes were that of an acute orchitis with massive neutrophilic and macrophage infiltrates into the connective tissue between seminiferous tubules. Meningitis and focal hemorrhages were observed in the brain and spinal cord. A hemadsorbing agent was also isolated from the blood of each of the four infected pigs using SPBMCs and the identity confirmed as the ASF virus by PCR as described under investigation of field cases above.
Table I: Rectal temperature of pigs experimentally infected with African swine fever

Table II: Clinical findings in experimentally infected pigs

Table III: Gross postmortem findings in experimentally infected pigs

Figure 2: Spleen with hemorrhage, necrosis and vasculitis (x 200).

Figure 3: Lymph node with edema, necrosis and vasculitis (x 200).
Discussion
The precise land area involved in the present outbreak was not known, but death of pigs was reported from all the settlements in a rural area of over 200 km2 with a human population of about 10,000. The crude oil spillage which coincided with the period of pig deaths, and which the pigs’ rearers suspected to be the source of intoxication, originated from an off-shore location but did not extend beyond 200 m inland. However death of pigs occurred in locations up to 15 km inland and no evidence of oil was seen on or inside any of the pigs examined. Hence hydrocarbon oil intoxication was ruled out as the cause of the widespread death of pigs.
All the experimentally infected pigs died 5 to 6 days p.i. The short and fatal course of the infection showed that the ASFV strain involved was highly virulent to naive pigs as is characteristic of virgin outbreaks (2, 10). On the basis of clinical signs and necropsy lesions, ASF may be confused with other septicemic diseases such as hog cholera, acute erysipelas, salmonellosis and African trypanosomosis caused by Trypanosoma simiae. Previous workers have shown that ASFV causes proliferation, and, later, degeneration and necrosis of lymphocytes and reticular cells (8). It is possible that the bacterial invasion of the spleen and lymph nodes observed in the field cases resulted from opportunistic bacterial infections following immune suppression as a consequence of extensive destruction of the cells of the recticulo-endothelial system by ASFV. The diagnosis of ASF was established in this investigation by amplification of a fragment of the structural protein VP72 of the virus from the tissues of both naturally and experimentally infected pigs using the PCR technique (13). Previous workers have shown that detection of this fragment of the VP72 of ASFV in tissues is specific for the diagnosis of the virus infection (5, 7).
There seemed to have been two likely sources of ASFV infection in the present study. Firstly, swill from the kitchens of the facilities of oil prospecting companies, which included pork products, commonly fed to pigs in the area of study, and garbage containing uncooked pork scraps with viable ASFV were major sources of the disease. Secondly, the disease might have spread from neighboring infected areas through importation of apparently healthy but infected animals incubating the disease, or indirectly by movements of contaminated personnel, equipment, and also by pig carcasses thrown into waterways. ASFV isolated from the present outbreak showed 100% similarity to the virus isolated from Lagos, Nigeria, earlier in the same year (13). It thus seemed that the infection was most likely spread from contiguous infected areas. In this respect the inter-connectivity of settlements along the Atlantic coast by waterways might have facilitated spread of ASF over a wide area. However, Penrith (14) is of the view that streams and rivers do not constitute a serious risk as large amounts of ASFV are required to infect pigs, and in water the virus generally becomes dispersed so that intake of the virus will be too low to cause the disease: in instances where ASF appeared to spread along the shores of a river, floating carcasses washed off the shore were likely to have been the source of the disease.
Due to the lack of implementation of a slaughter and compensation policy in Nigeria, ASF is likely to become established as an enzootic disease as in several other sub-Saharan African countries, especially since effective vaccines are non-existent (11). The finding of orchitis at histopathology further suggests that ASF may adversely influence reproductive performance in enzootic areas.
Acknowledgments
The authors wish to thank Prof. R.A. Oderinde and Dr P.A. Ajayi for their help during field visits, Prof. B.O. Ikede for the production of photomicrographs and Chevron Nigeria Ltd for logistics. Molecular biology work in the Department of Virology was carried out using equipment donated by the Deutscher Akademischer Austausch Dienst and the Alexander von Humboldt Stiftung to Dr Odemuyiwa and Prof. Olaleye, respectively.
References
1. ALTSCHUL S.F., MADDEN T.L., SCHAFFER A.A., ZHANG J., ZHANG Z., MILLER W., LIPMAN D.J., 1997. Gapped BLAST PSI-BLAST 1997. A new generation of protein database search programs. Nucleic Acids Res., 25: 3389-3402.
2. Animal health manual No 9, 2004. Recognizing African swine fever. A field manual. Rome, Italy, FAO, p. 1-30.
3. BARROW G.H., FELTHAM R.K.A., 1993. Cowan and Steel’s manual for identification of medical bacteria, 3rd Edn. Cambridge, UK, Cambridge University Press, 331 p.
4. DEKOCK G., ROBINSON E.M., KEPPEL J.G., 1940. Swine fever in South Africa. Onderst. J. vet. Sci. Anim. Ind., 14: 31.
5. KING D.P., REID S.M., HUTCHINGS G.H., GRIERSON S.S., WIKINSON P.J., DIXSON L.K., BASTOS A.D., DREW T.W., 2003. Development of a TaqMan PCR assay with internal amplification control for the detection of African swine fever virus. J. Virol. Meth., 107: 53-61.
6. KLOOS W.E., BANNERMAN T.L., 1999. Staphylococci and Micrococcus. In: Murray P.K., Baron E.J., Pfaller M.A., Tenover F.C., Yolken R.Y., Eds, Manual of clinical microbiology, 7th Edn. Washington DC, USA, American Society of Microbiology Press, p. 264-282.
7. LEITAO A., MALUR A., CORNELIS P., MARTINS C.L., 1998. Identification of a 25-aminoacid sequence from the major Africa swine fever virus structural protein VP72 recognised by porcine cytotoxic T lymphocytes using a lipoprotein based expression system. J. Virol. Meth., 75: 113-119.
8. MCDANIEL H.A., 1986. African swine fever. In: Diseases of swine, 5th Edn. Ames, Iowa, USA, Iowa State University Press, p. 237-245.
9. MONTGOMERY R.E., 1921. On a form of swine fever occurring in British East Africa (Kenya Colony). J. comp. Pathol., 34: 159-191.
10. MEBUS C.A., 1988. African swine fever. Adv. Virus Res., 35: 251-268.
11. MURPHY F.A., GIBBS E.P.J., HORZINEK M.C., STUDDERT M.J., 1999. Viral taxonomy and nomeclature. In: Murphy F.A., Ed., Veterinary virology. Millbrae, CA, USA, California Academic Press, p. 22-30.
12. MURRAY M., MURRAY P.K., MCINTYRE W.I.M., 1977. An improved parasitological technique for diagnosis of African trypanosomiasis. Trans. R. Soc. trop. Med. Hyg., 7: 325-326.
13. ODEMUYIWA S.O., ADEBAYO I.A., AMMERLAAN W., AJUWAPE A.T.P., ALAKA O.O., OYEDELE O.I., SOYELU K.O., OLALEYE D.O., OTESILE E.B., MULLER C.P., 2000. An outbreak of African swine fever in Nigeria: Virus isolation and molecular characterization of the VP72 gene of a first isolate from West Africa. Virus Genes, 20: 139-142.
14. PENRITH M.L., 1998. Overview of African swine fever. Rome, Italy, FAO.
15. PLOWRIGHT W., PARKER J., PIERCE M.A., 1969. The epizootiology of African swine fever in Africa. Vet Rec., 85: 668-674.
16. VELHO E.L., 1956. Observations sur la peste porcine en Angola. Bull. Off. int. Epizoot., 46 : 335.
17. WILKINSON P.J., 1984. The persistence of African swine fever in Africa and the Mediterranean. Prev. vet. Med., 2: 71-82.
18. WILKINSON P.J., DONALSON A.I., GREIG A., BRUCE W., 1977. Transmission studies with African swine fever infection of pigs with air borne virus. J. comp. Pathol., 87: 487-495.
For further information





Comments
CIRAD © 2007 (All rights reserved) - Disclaimer stating - Page updated : 05/04/2007
